Publikationen
[59]
Facile modular synthesis of jasmonoyl-L-isoleucine analogs possessing a pyrazolidin-3-one core
S. Vizcaíno Páez, D. Durango, C. J. Müller, M. Breuning, W. Quiñones Fletcher
RSC Adv., 2024, 14, 3790–3797
DOI: 10.1039/D3RA07887F

[58]
Biological activities of 4H-thiochromen-4-one 1,1-dioxide derivatives against tropical disease parasites: A target-based drug design approach
C. Ortiz, M. Breuning, S. Robledo, F. Echeverri, E. Vargas a, W. Quiñones
Heliyon, 2023, 9, e17801
DOI: 10.1016/j.heliyon.2023.e17801
[57]
Sterically Encumbered Coordination Sites: Iron(II) Complexes of Jäger-type ligands with a Terphenyl Backbone
A. Dürrmann, G. Hörner, S. Wagner, M. Breuning, B. Weber
Z. Anorg. Allg. Chem. 2021, 647, 1–11
DOI: 10.1002/zaac.202100196
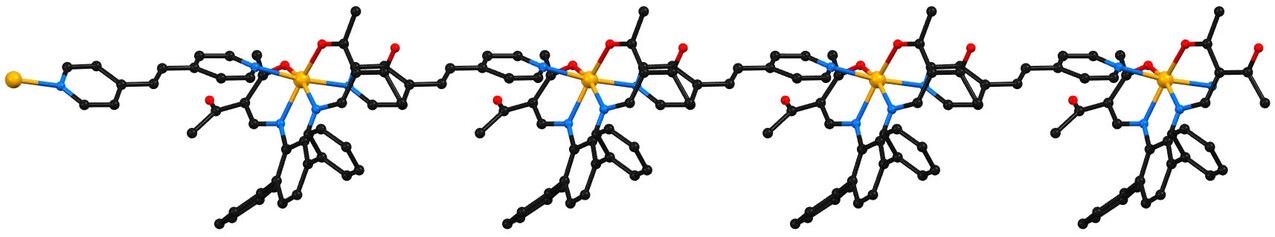
[56]
Diastereodivergent Synthesis of the Quinolizidine-Indolizidine Alkaloids of the Leontidine/Camoensine Family
S. Wagner, S. Sigl, M. Schenkl, M. Breuning
Eur. J. Org. Chem. 2021, 2021, 2498–2505
DOI: 10.1002/ejoc.202100270

[55]
An Artificial Cofactor Catalyzing the Baylis-Hillman Reaction with Designed Streptavidin as Protein Host
H. Lechner, V. R. Emann, M. Breuning, B. Höcker
ChemBioChem 2021, 22, 1573–1577
DOI: 10.1002/cbic.202000880

[54]
Enantioselective addition of diethylzinc to aldehydes catalyzed by 5-cis-substituted proline derivatives
F. Prause, S. Wagner, M. Breuning
Tetrahedron 2019, 75, 1. 94–101.
DOI: 10.1016/j.tet.2018.11.030

[53]
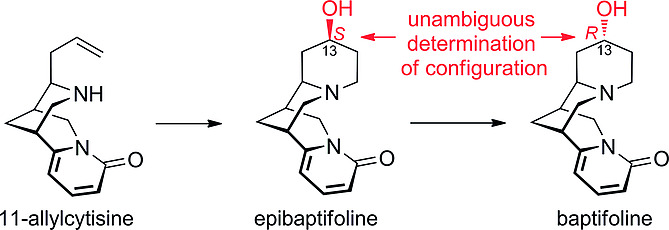
The Hydroxylated, Tetracyclic Bisquinolizidine Alkaloids Baptifoline and Epibaptifoline: Enantioselective Synthesis and Unambiguous Assignment of their Configuration at C‐13
J. Goller, C. B. Hübschle, M. Breuning
Eur. J. Org. Chem. 2019, 895–899
DOI: 10.1002/ejoc.201801126
[52]
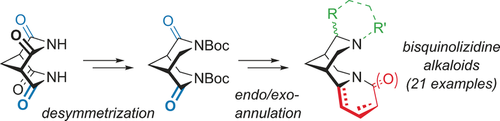
Die enantioselektive Totalsynthese von Bischinolizidin-Alkaloiden: Ein modularer "Inside-Out"-Zugang
D. Scharnagel, J. Goller, N. Deibl, W. Milius, M. Breuning
Angew. Chem. 2018, 130, 2456-2460.
DOI: 10.1002/ange.201712852
Angew. Chem. Int. Ed. 2018, 57, 2432-2435.
DOI: 10.1002/anie.201712852
Featured in Synfacts 2018, 14, 342.
DOI: 10.1055/s-0037-1609332
[51]
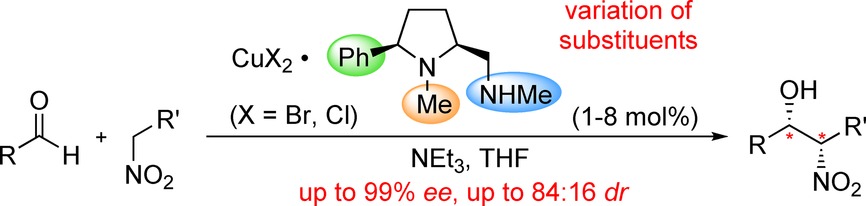
Evaluation of 5-cis-Substituted Prolinamines as Ligands in Enantioselective, Copper-Catalyzed Henry Reactions
J. Kaldun, F. Prause, D. Scharnagel, F. Freitag, M. Breuning
ChemCatChem 2016, 8, 1846-1856
DOI: 10.1002/cctc.201600240
[50]
Enantioselective borane reduction of ketones catalyzed by tricyclic 1,3,2-oxazaborolidines
J. Kaldun, A. Krimalowski, M. Breuning
Tetrahedron Lett. 2016, 57, 2492-2495
DOI: 10.1016/j.tetlet.2016.04.091
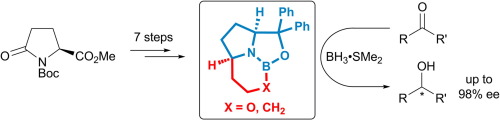
[49]
The First Modular Route to Core-Chiral Bispidine Ligands and Their Application in Enantioselective Copper(II)-Catalyzed Henry Reactions
D. Scharnagel, A. Müller, F. Prause, M. Eck, J. Goller, W. Milius, M. Breuning
Chem. Eur. J. 2015, 21, 12488–12500
DOI: 10.1002/chem.201502090
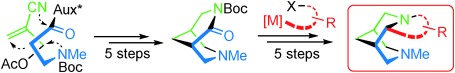
[48]
In-depth structure–selectivity investigations on asymmetric, copper-catalyzed oxidative biaryl coupling in the presence of 5-cis-substituted prolinamines
F. Prause, B. Arensmeyer, B. Fröhlich, M. Breuning
Catal. Sci. Technol. 2015, 5, 2215–2226
DOI: 10.1039/C4CY01676A
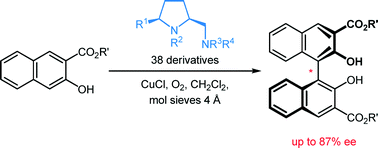
[47]
Flexible and Modular Syntheses of Enantiopure 5-cis-Substituted Prolinamines from L-Pyroglutamic Acid
F. Prause, J. Kaldun, B. Arensmeyer, B. Wennemann, B. Fröhlich, D. Scharnagel, M. Breuning
Synthesis 2015, 47, 575–580
DOI: 10.1055/s-0034-1379457
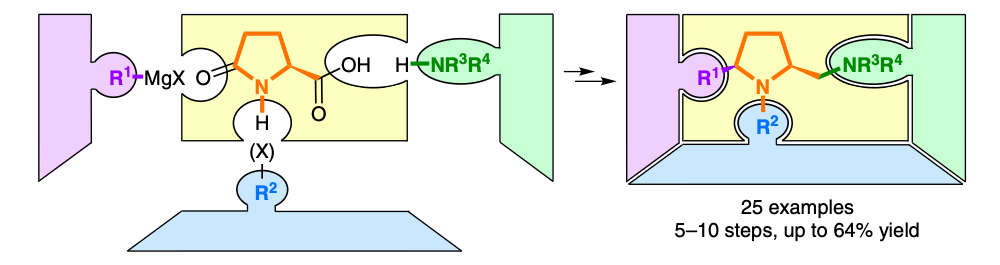
[46]
(2S,5R)-2-Methylaminomethyl-1-methyl-5-phenylpyrrolidine, a chiral diamine ligand for copper(II)-catalysed Henry
reactions with superb enantiocontrol
D. Scharnagel, F. Prause, J. Kaldun, R. G. Haase, M. Breuning
Chem. Commun. 2014, 50, 6623–6625
DOI: 10.1039/C4CC02429J
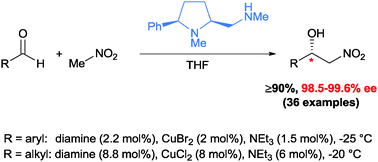
[45]
5-Substituted Derivatives of the Tricyclic (+)-Sparteine Surrogate in the Enantioselective Lithiation/Stannylation of an
O-Alkyl Carbamate
M. Breuning, D. Hein
Eur. J. Org. Chem. 2013, 7575–7582
DOI: 10.1002/ejoc.201300987
[44]
Theoretical and spectroscopic studies on the conformational equilibrium of 9-oxabispidines in solution
M. Breuning, A. Paasche, M. Steiner, S. Dilsky, V. H. Gessner, C. Strohmann, B. Engels
J. Mol. Struct. 2011, 1005, 178–185
DOI: 10.1016/j.molstruc.2011.08.047
[43]
Übersichtsartikel: Atroposelective Total Synthesis of Axially Chiral Biaryl Natural Products
G. Bringmann, T. Gulder, T. A. M. Gulder, M. Breuning
Chem. Rev. 2011, 111, 563–639
DOI: 10.1021/cr100155e
[42]
Axialchirale Biaryle
M. Breuning
GIT Labor-Fachzeitschrift 2010, 524–526
[41]
Lactide polymerization with 9-oxabispidine zinc complexes
J. Börner, U. Flörke, S. Herres-Pawlis, A. Döring, D. Kuckling, M. D. Jones, M. Steiner, M. Breuning, M. Steiner
Inorg. Chem. Commun. 2010, 13, 369–371
DOI: 10.1016/j.inoche.2009.12.024
[40]
Enantioselective synthesis of tricyclic amino acid derivatives based on a rigid 4-azatricyclo[5.2.1.02,6]decane skeleton
M. Breuning, T. Häuser, C. Mehler, C. Däschlein, C. Strohmann, A. Oechsner, H. Braunschweig
Beilstein J. Org. Chem. 2009, 5, No. 81
DOI: 10.3762/bjoc.5.81
[39]
Bridgehead-Lithiated 9-Oxabispidines
M. Breuning, M. Steiner, C. Hörl, P. Maier
Synlett 2009, 2749–2754
[DOI: 10.1055/s-0029-1217999]
[38]
Chiral 2-endo-Substituted 9-Oxabispidines: Novel Ligands for Enantioselective Copper(II)-Catalyzed Henry Reactions
M. Breuning, D. Hein, M. Steiner, V. H. Gessner, C. Strohmann
Chem. Eur. J. 2009, 15, 12764–12769
[DOI: 10.1002/chem.200901789]
[37]
A Novel One-Pot Procedure for the Stereoselective Synthesis of alpha-Hydroxy Esters from Ortho Esters
M. Breuning, T. Häuser, E.-M. Tanzer
Org. Lett. 2009, 11, 4032–4035
[DOI: 10.1021/ol901214n]
[36]
A Flexible Route to Chiral 2-endo-Substituted 9-Oxabispidines and Their Application in the Enantioselective Oxidation
of Secondary Alcohols
M. Breuning, M. Steiner, C. Mehler, A. Paasche, D. Hein
J. Org. Chem. 2009, 74, 1407–1410
[DOI: 10.1021/jo802409x]
[35]
Enantioselective total synthesis of the tricyclic 9-oxabispidine (1R,2S,9S)-11-methyl-13-oxa-7,11-diazatricyclo[7.3.1.02,7]tridecane
M. Breuning, M. Steiner
Tetrahedron: Asymmetry 2008, 19, 1978–1983
[DOI: 10.1016/j.tetasy.2008.08.002]
[34]
ÜBERSICHTSARTIKEL: Chiral Bispidines
M. Breuning, M. Steiner
Synthesis 2008, 2841–2867
[DOI: 10.1055/10.1055/s-2008-1067241]
[33]
First asymmetric synthesis of a C2-symmetric 2-endo,6-endo-disubstituted bispidine
M. Breuning, D. Hein
Tetrahedron: Asymmetry 2007, 18, 1410–1418
[DOI: 10.1016/j.tetasy.2007.06.010]
[32]
Enantioselective synthesis of 2-phenyl-9-oxabispidines
M. Breuning, M. Steiner
Synthesis 2007, 1702–1706
[DOI: 10.1055/s-2007-966040]
[31]
Efficient one-pot synthesis of enantiomerically pure 2-(hydroxymethyl)morpholines
M. Breuning, M. Winnacker, M. Steiner
Eur. J. Org. Chem. 2007, 2100–2106
[DOI: 10.1002/ejoc.200601006]
[30]
Unprecedented formation of stable ketene-N,O-acetals and their rearrangement under basic conditions
M. Breuning, T. Häuser
Tetrahedron 2007, 63, 934–940
[DOI: 10.1016/j.tet.2006.11.033]
[29]
Convenient multigram synthesis of (R)-homopipecolic acid methyl ester
M. Breuning, M. Steiner
Synthesis 2006, 1386–1389
[DOI: 10.1055/s-2006-926419]
[28]
Übersichtsartikel: Atroposelective Synthesis of Axially Chiral Biaryl Compounds
G. Bringmann, A. J. Price Mortimer, P. A. Keller, M. J. Gresser, J. Garner, M. Breuning
Angew. Chem. 2005, 117, 5518–5563; Angew. Chem. Int. Ed. 2005, 44, 5384–5427
[DOI: 10.1002/ange.200462661], [DOI: 10.1002/anie.200462661]
[27]
Synthesis and antitrypanosomal activity of 2-aminomethyl-1-(2-oxyphenyl)naphthalenes
G. Bringmann, R.-M. Pfeifer, P. Schreiber, K. Hartner, N. Kocher, R. Brun, K. Peters, E.-M. Peters, M. Breuning
Tetrahedron 2004, 60, 6335–6344
[DOI: 10.1016/j.tet.2004.05.076]
[26]
The 'lactone method': enantioselective preparation of novel P,N-biaryl ligands and their use in the synthesis of the
biarylic alkaloids, ancistrotanzanine B and ancistroealaine A
G. Bringmann, R.-M. Pfeifer, P. Schreiber, K. Hartner, M. Schraut, M. Breuning
Tetrahedron 2004, 60, 4349–4360
[DOI: 10.1016/j.tet.2003.12.070]
[25]
Structure of trans-Isoshinanolone in the Crystal and in Solution
G. Bringmann, R.-M. Pfeifer, M. Breuning, M. Reichert, K. Messer, M. Schraut, G. Toth
Z. Naturforsch. B, Chem. Sci. 2004, 59, 100–105
[DOI: 10.1515/znb-2004-0114]
[24]
Synthesis of Enantiopure Axially Chiral C3-Symmetric Tripodal Ligands and Their Application as Catalysts in the Asymmetric Addition of Dialkylzinc to Aldehydes
G. Bringmann, R.-M. Pfeifer, C. Rummey, K. Hartner, M. Breuning
J. Org. Chem. 2003, 68, 6859–6863
[DOI: 10.1021/jo034697t]
[23]
Atropo-enantioselective synthesis of a C3-symmetric tripodal ligand with three axially chiral biaryl subunits
G. Bringmann, M. Breuning, R.-M. Pfeifer, P. Schreiber
Tetrahedron: Asymmetry 2003, 14, 2225–2228
[DOI: 10.1016/S0957-4166(03)00406-3]
[22]
Asymmetric Synthesis of (M)-2-Hydroxymethyl-1-(2-hydroxy-4,6-dimethylphenyl)naphthalene
G. Bringmann, M. Breuning, P. Henschel, J. Hinrichs
Org. Synth. 2002, 79, 72–83; Org. Synth. 2004, Coll. Vol. 10, 448–459
[DOI: 10.1002/0471264180.os079.09]
[21]
ÜBERSICHTSARTIKEL: The directed synthesis of axially chiral ligands, reagents, catalysts, and natural products
through the 'lactone methodology'
G. Bringmann, S. Tasler, R.-M. Pfeifer, M. Breuning
J. Organomet. Chem. 2002, 661, 49–65
[DOI: 10.1016/S0022-328X(02)01819-3]
[20]
ÜBERSICHTSARTIKEL: The lactone concept – a novel approach to the metal-assisted atroposelective construction
of axially chiral biaryl systems
G. Bringmann, M. Breuning, R.-M. Pfeifer, W. A. Schenk, K. Kamikawa, M. Uemura
J. Organomet. Chem. 2002, 661, 31–47
[DOI: 10.1016/S0022-328X(02)01804-1]
[19]
Catalytic Enantioselective Diels-Alder Reactions of 1,4-Quinone Monoketals
M. Breuning, E. J. Corey
Org. Lett. 2001, 3, 1559–1562
[DOI: 10.1021/ol015852y]
[18]
Atropisomerization Barriers of Configurationally Unstable Biaryl Compounds, Useful Substrates for Atroposelective Conversions to Axially Chiral Biaryls
G. Bringmann, M. Heubes, M. Breuning, L. Göbel, M. Ochse, B. Schöner, O. Schupp
J. Org. Chem. 2000, 65, 722–728
[DOI: 10.1021/jo9913356]
[17]
ÜBERSICHTSARTIKEL: The Lactone Concept: An Efficient Pathway to Axially Chiral Natural Products and Useful Reagents
G. Bringmann, M. Breuning, S. Tasler
Synthesis 1999, 525–558
[DOI: 10.1055/s-1999-3435]
[16]
Synthesis of Axially Chiral Biaryls by Atropo-Diastereoselective Cleavage of Configurationally Unstable Biaryl
Lactones with Menthol-Derived O-Nucleophiles
G. Bringmann, M. Breuning, R. Walter, A. Wuzik, K. Peters, E.-M. Peters
Eur. J. Org. Chem. 1999, 3047–3055
[DOI: 10.1002/(SICI)1099-0690(199911)1999:11<3047::AID-EJOC3047>3.0.CO;2-O]
[15]
Atropo-Diastereoselective Cleavage of Configurationally Unstable Biaryl Lactones with Alkali Metal Activated Primary 1-Arylethylamines
G. Bringmann, M. Breuning, S. Tasler, H. Endress, C. L. J. Ewers, L. Göbel, K. Peters, E.-M. Peters
Chem. Eur. J. 1999, 5, 3029–3038
[DOI: 10.1002/(SICI)1521-3765(19991001)5:10<3029::AID-CHEM3029>3.0.CO;2-5]
[14]
Atropo-enantioselective synthesis of an axially chiral C1-symmetric phosphine ligand and its application in the
asymmetric hydrosilylation of styrenes
G. Bringmann, A. Wuzik, M. Breuning, P. Henschel, K. Peters, E.-M. Peters
Tetrahedron: Asymmetry 1999, 10, 3025–3031
[DOI: 10.1016/S0957-4166(99)00299-2]
[13]
Crystal structure of 1-(2-hydroxy-4,6-dimethoxyphenyl)-2-propyl naphthoate, C6H10(COOC3H7)[C6H2(OCH3)2OH]
K. Peters, E.-M. Peters, M. Breuning, G. Bringmann
Z. Kristallogr. NCS 1999, 214, 251–252
[12]
Atropo-enantioselective reduction of configurationally unstable biaryl lactones with BINAL-H
G. Bringmann, M. Breuning
Tetrahedron: Asymmetry 1999, 10, 385–390
[DOI: /10.1016/S0957-4166(98)00503-5]
[11]
The Atropo-Enantioselective Reduction of Configurationally Unstable Biaryl Hydroxy Aldehydes – A Novel Approach
to Axially Chiral Biaryls
G. Bringmann, M. Breuning
Synlett 1998, 634–636
[DOI: 10.1055/s-1998-1728]
[10]
The ortho-Hydroxy-ortho'-Formyl Biaryl / Lactol Equilibrium: Quantumchemical Studies on Structure and Dynamics
G. Bringmann, D. Vitt, J. Kraus, M. Breuning
Tetrahedron 1998, 54, 10691–10698
[DOI: 10.1016/S0040-4020(98)00619-X]
[9]
Biaryl Hydroxy Aldehydes as Intermediates in the Metal-Assisted Atropo-Enantioselective Reduction of Biaryl Lactones: Structures and Aldehyde-Lactol Equilibria
G. Bringmann, M. Breuning, H. Endress, D. Vitt, K. Peters, E.-M. Peters
Tetrahedron 1998, 54, 10677–10690
[DOI: 10.1016/S0040-4020(98)00618-8]
[8]
Calculation of Structures, Activities and Spectroscopic Properties of Metal-Activated Molecules
G. Bringmann, M. Breuning, S. Busemann, J. Kraus, C. Rummey, R. Stowasser, D. Vitt, W. Kiefer, C. Fickert, T. Linker, F. Rebien, W. Malisch, S. Möller, J. Sundermeyer, G. Wahl in
Selective Reactions of Metal-Activated Molecules (Eds.: H. Werner, P. Schreier), Vieweg, Braunschweig, 1998, S. 295–296
[7]
Metal-Asisted Synthesis and Application of Axially Chiral Biaryl Systems
G. Bringmann, M. Breuning, S. Busemann, J. Hinrichs, T. Pabst, R. Stowasser, S. Tasler, A. Wuzik, W. A. Schenk, J. Kümmel, D. Seebach, G. Jaeschke in
Selective Reactions of Metal-Activated Molecules (Eds.: H. Werner, P. Schreier), Vieweg, Braunschweig, 1998, S. 141–145
[6]
Crystal structure of 2-bromomethyl-1-(2-benzyloxy-4,6-dimethylphenyl)naphthalene, C10H6(CH2Br)C6H2(CH3)2OCH2C6H5
K. Peters, E.-M. Peters, M. Breuning, G. Bringmann
Z. Kristallogr. NCS 1998, 213, 555–556
[5]
Enantioselective addition of diethylzinc to aldehydes using novel axially chiral 2-aminomethyl-1-(2'-hydroxyphenyl) naphthalene catalysts
G. Bringmann, M. Breuning
Tetrahedron: Asymmetry 1998, 9, 667–679
[DOI: 10.1016/S0957-4166(98)00020-2]
[4]
Crystal structure of 1-ethyl-1-[1-(2-hydroxy-4,6-dimethylphenyl)-2-naphthyl)]propan-1-ol, [C6H2(CH3)2OH][C(OH)(C2H5)2]C10H6
K. Peters, E.-M. Peters, M. Breuning, G. Bringmann
Z. Kristallogr. NCS 1998, 213, 345–346
[3]
Crystal structure of methyl 1-(2-hydroxy-4,6-dimethylphenyl)naphthyl)-2-carboxylate, [C6H2(CH3)2OH](COOH3)C10H6
K. Peters, E.-M. Peters, M. Breuning, O. Schupp, A. Wuzik, G. Bringmann
Z. Kristallogr. NCS 1998, 213, 339–340
[2]
Cooperative Reactivity of Unsupported Early-Late Heterobimetallics: Ring Opening and Subsequent Decarbonylation
of Biaryllactones
A. Schneider, L.H. Gade, M. Breuning, G. Bringmann, I.J. Scowen, M. McPartlin
Organometallics 1998, 17, 1643–1645
[DOI: 10.1021/om980007d]
[1]
Resolution of Racemic Carboxylic Acid Derivatives by Ti-TADDOLate Mediated Esterification Reactions – A General
Method for the Preparation of Enantiopure Compounds
D. Seebach, G. Jaeschke, K. Gottwald, K. Matsuda, R. Formisano, D.A. Chaplin, M. Breuning, G. Bringmann
Tetrahedron 1997, 53, 7539–7556
[DOI: 10.1016/S0040-4020(97)00456-0]
Buchbesprechungen
[1]
Modern Aldol Reactions, edited by Rainer Mahrwald
M. Breuning
Angew. Chem. 2005, 117, 2089; Angew. Chem. Int. Ed. 2005, 44, 2053
[DOI: 10.1002/ange.200385243], [DOI: 10.1002/anie.200385243]
[2]
Modern Tools for the Synthesis of Complex Bioactive Molecules, edited by Janine Cossy and Stellios Arseniyadis
M. Breuning
Angew. Chem. 2013, 125, 6704; Angew. Chem. Int. Ed. 2013, 52, 6574
[DOI: 10.1002/ange.201303814], [DOI: 10.1002/anie.201303814]
Patente
[1]
Cyclopentene Derivatives
W. Hübsch, M. Breuning, G. Schmidt, B. Albrecht, E. Perzborn, C. Faeste, L. Bärfacker
PCT Int. Appl. WO 2003080553, 2003
